
Topical Finasteride
with SiloxysSystem™ Gel
(Delivered every 3 months)
Topical finasteride from XYON treats hair loss at its source. Stop hair thinning and promote regrowth. Formulated with finasteride, a medication clinically-proven to be effective at treating hair loss. Formulated with patented gel technology, designed to reduce absorption of finasteride into the body and lower the risk of side effects. Developed by doctors. Dermatologist tested.
How the Treatment Works
Finasteride treats hair loss by lowering levels of a hormone called DHT, widely considered to be the main cause of pattern hair loss in men. It's delivered with SiloxysSystem™ Gel that is designed to target medication to hair follicles and lower the risk of sexual side effects.
Ingredients
- 2.5% Finasteride: Lowers levels of DHT, widely considered a primary cause of hair loss in men. It's an example of a medication called a 5-alpha reductase inhibitor.
- SiloxysSystem™ Gel: Patented gel designed to maximize results and minimize absorption of medications into the body.
How to Access a Prescription
- Begin a complementary virtual intake to be assessed by a specialist physician.
- A specialist physician will review your profile and info to determine your eligibility.
- If you're issued a prescription, we'll deliver it to you in discreet packaging, right to your doorstep.
* Topical finasteride is a compounded medication and has not been approved by the FDA. Only available if prescribed after an online consultation with a licensed healthcare provider.

Before and after
Topical finasteride results
Members of the XYON community are sharing their hair regrowth results using topical finasteride. See what others have been able to achieve with our performance hair loss treatments.
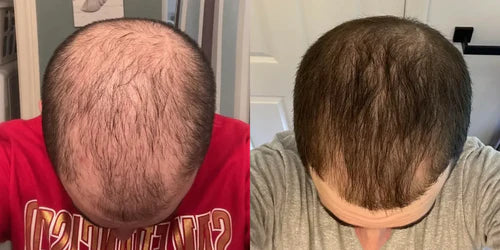 3 Months
3 Months

Chris, 23
"I've seen incredible results with finasteride and minoxidil after 4 months. I was basically bald when I started, but now my hair has shown crazy improvement, especially considering it often takes 4-6 months to see results. My crown is almost completely filled, and I'm excited to see even more progress."
Taking Topical Finasteride for overall thinning and thinning at the crown
Photo Taken 3 Months Into XYON Treatment
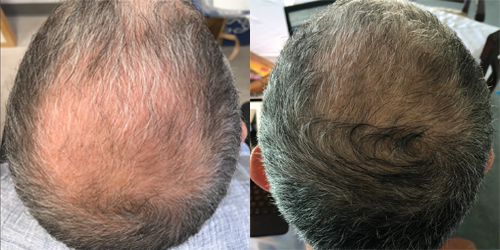 2 Months
2 Months
Michael
"This stuff is incredible. I have been using it for 3 years now and I essentially have a full head of hair now. Here are the before an agree pictures from a couple of years ago - about two months after I began using Xyon’s product. (Just read the other views and came back to add this - no side effects!)"
Taking Topical Finasteride for overall thinning and thinning at the crown
Photo Taken 2 Months Into XYON Treatment
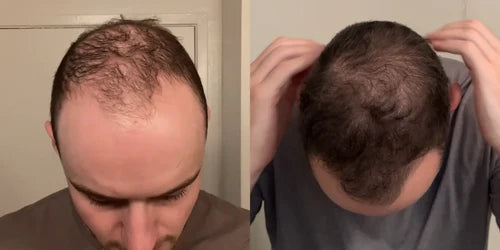 3 Months
3 Months

Zeph, 28
"I used to shave my head because my hair was so thin and frail. But XYON not only restored my hair but also my confidence. The results speak for themselves. I hope others can experience the same positive changes I have."
Taking Topical Finasteride for overall thinning and receding hairline
Photo Taken 3 Months Into XYON Treatment
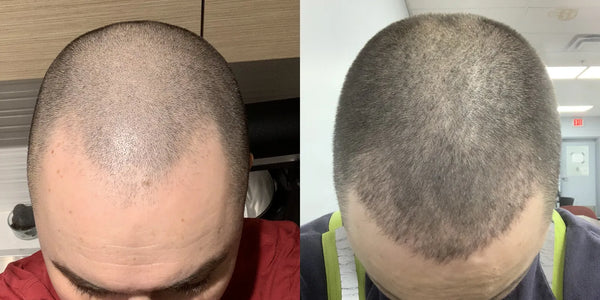 3 Months
3 Months
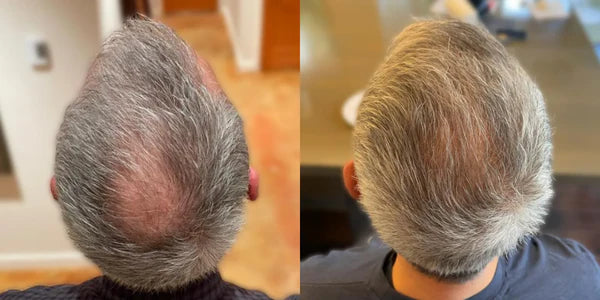
MD, 35
Taking Topical Finasteride for a receding hairline
Photo Taken 3 Months Into XYON Treatment
 3 Months
3 Months
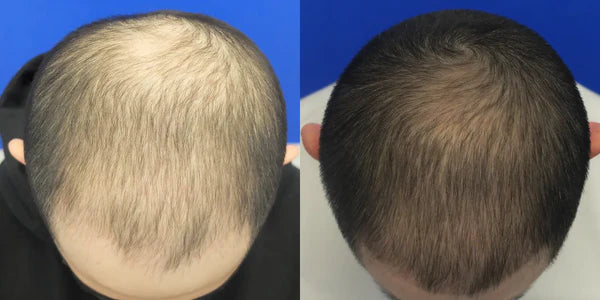
DX
Taking Topical Finasteride for thinning at the crown
Photo Taken 3 Months Into XYON Treatment
 3 Months
3 Months
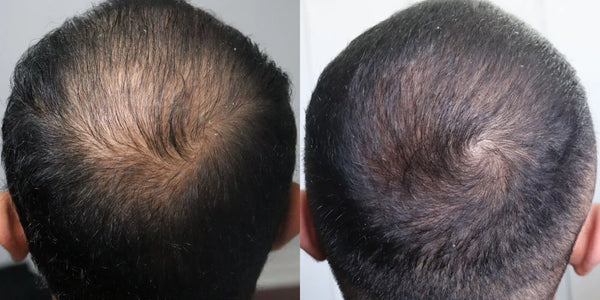
VYX, 28
Taking Topical Finasteride for overall thinning
Photo Taken 3 Months Into XYON Treatment
 3 Months
3 Months
AH, 28
Taking Topical Finasteride for thinning at the crown
Photo Taken 6 Months Into XYON Treatment
US Pat. no. 11,786,466
SiloxysSystem™ Gel
Our patented gel technology is here to shake things up. Designed to deliver medications over time, this uniquely formulated gel helps limit absorption of ingredients into the body. It's a potentially safer way of managing thinning hair.
Targeted application
With SiloxySystem™ Gel, the medication is applied directly to the scalp, targeting the exact area where it’s needed most. Unlike sprays that often spread unevenly or pills that work systemically, this precision helps maximize effectiveness and focus the treatment on your hair follicles.
Controlled release
The SiloxysSystem™ Gel delivers medication gradually over time, ensuring consistent and effective treatment throughout the day. Unlike sprays and pills, this controlled release minimizes peaks and troughs in absorption, helping your scalp get just the right amount of medication when it needs it.
Minimize absorption
Traditional pills send medication throughout your entire body, which can lead to side effects. The SiloxysSystem™ Gel is designed to limit the amount of medication absorbed into the bloodstream, ensuring more of the active ingredients stay on your scalp where they can work best.
Topical finasteride: highlights from XYON's clinical study.
Our own clinical studies have shown that topical finasteride may be an effective option for treating male pattern hair loss, with a much smaller impact on blood DHT levels and more localization to the skin, compared to the oral medication.

How topical finasteride works.
XYON topical finasteride works by harnessing the power of a 5-alpha reductase inhibitor to prevent testosterone from being converted into DHT. Lowering DHT levels is essential to slowing the progression of hair loss and giving hair follicles a chance to regrow. Start an online intake with a doctor today to see if prescription topical finasteride is right for you. You can also learn more about finasteride's mechanism of action by visiting our medical library.
Does topical finasteride work for men?
Clinical studies have shown that topical finasteride can be an effective treatment option for pattern hair loss, with a lower risk of adverse effects such as sexual dysfunction and decreased libido.
less finasteride absorbed into the blood compared to oral finasteride after 24 weeks of treatment.
of patients felt that topical finasteride was highly effective at treating their hair loss.
less of an impact on blood DHT levels compared to the oral medication after 24 weeks of treatment.
Designed to reduce the side effects of finasteride.
Although it's considered to be one of the more effective treatments for male hair loss, oral finasteride has been linked to potential sexual side effects and mood changes, as well as an increased risk of developing some prostate cancers (Hirshburg et al., 2016). This is why our patented SiloxysSystem™ technology was designed to lower the risk of these possible side effects.
Learn more
Topical Gel with SiloxysSystem™

Topical Solution

Oral Medications

Topical Gel with SiloxysSystem™

Topical Solution

Oral Medications
A gel you apply with your fingertips
A liquid solution you apply with a dropper
A pill you swallow
Once per day, or as indicated in your prescription
Once per day, or as indicated in your prescription
Once per day, or as indicated in your prescription
Designed to deliver hair loss medications to the scalp using exclusive controlled and time-released technology that maximizes efficacy and minimizes side effects
Lightweight, fast-absorbing formula delivers hair loss medications to the scalp without residue
Absorbed into the body and travel through the bloodstream to the hair follicles where they take effect
The least amount of absorption of medication and potentially lowest risk of side effects
Generally less absorption compared to oral medications, potentially lower risk of side effects
Of the three options, the highest amount of absorption of medication, which can increase the risk of side effects
Starting at $99 per month
$68 per month
Starting at $35 per month
Higher dosage (2.5% finasteride, 2.5 % finasteride + 3% minoxidil, or 2% dutasteride)
Low dose (0.25% finasteride + 6% minoxidil)
Standard FDA-approved dose (1mg finasteride)
Yes, part the hair to reveal scalp skin and use fingertips to apply the gel to affected areas only. No-drip, non-greasy formula.
Yes, part the hair to reveal scalp skin and split the amount of solution in your dropper between affected areas only. Lightweight, fast-absorbing formula.
Yes, take with water with or without food.
Yes
Yes
Yes
How to use XYON Topical Finasteride.
Here are some tips to help you make the most of your topical hair loss treatment.
- Each XYON bottle has a built-in safety lock. Turn the safety lock to the open position and press down once to dispense a pre-measured amount (1 mL) of treatment.
- Focus application of XYON topical finasteride to the affected areas of the scalp. If you have long or curly hair, you can try parting your hair to better access the scalp skin. Gently massage the treatment into the scalp for 20-30 seconds.
- You're done. Style your hair as usual, but make sure that you wash your hands with soap and warm water after applying and/or touching the gel.
For your safety and to maximize the success of your treatment, it's important to follow the dosing instructions provided to you by your prescribing doctor. We typically recommend applying your topical treatment first thing in the morning to allow it to sit undisturbed on the scalp for as long as possible (4-8 hours is ideal). Avoid applying before bed to lower the risk of transfer to pillows and/or sheets.
Missed a dose? It happens. Simply resume usual application as soon as possible. Don't double up on missed doses or change your dosing frequency without first consulting with your prescribing doctor.
Some people may experience increased shedding in the first few weeks or months after starting a new hair loss treatment like topical finasteride. In many cases, this shedding will resolve with time. If it worsens or doesn't stop, we recommend notifying your prescribing doctor. Visit our page on on finasteride shedding to learn more.
Visit the XYON medical library.
Our curated selection of medically-reviewed articles and resources on men's health and hair loss was created with you in mind. Stay up-to-date on the topics and issues that matter the most.
Frequently asked questions about topical finasteride.
Here are some common questions we get asked about topical finasteride.
Can I use finasteride with other hair loss treatments?
In some cases, yes. Depending on your hair loss, a doctor may recommend combining finasteride with a complementary treatment such as minoxidil, to help increase the likelihood of treatment success.
Of note, there have been clinical studies conducted that have shown that taking finasteride and minoxidil together may be more effective at re-growing hair than finasteride on its own.
If you're wondering whether you need another treatment in addition to finasteride, we recommend consulting with a doctor.
When can I expect results from using topical finasteride?
Generally speaking, it takes about 6 months of consistent treatment with topical finasteride before you'll see visible improvements. For some patients, this process could take longer (e.g. up to a year). Re-growing hair is a marathon, not a sprint.
What is the concentration of finasteride?
The concentration of finasteride in our topical formulation was chosen based on a series of clinical investigations. In the end, 2.5% finasteride in our topical gel struck a balance between safety and efficacy.
It's important to understand that the strength of the ingredients in any topical formulation depends on both the amount of ingredient present and the characteristics of the gel base. Our gel was specifically designed to help reduce systemic absorption. This is why doctors are able to prescribe higher concentrations to help address the needs and concerns of their patients.
Does finasteride require a prescription?
All forms of finasteride require a prescription.
XYON topical finasteride is a compounded medication that is only available with a prescription following an online intake with a licensed physician.
Is topical finasteride better than oral finasteride?
The answer to this question depends on the individual. A recent review of the relative efficacy of common hair loss treatments found that oral finasteride and topical finasteride work similarly well. The biggest difference is in the risk of experiencing side effects. Generally speaking, topical finasteride has been associated with a lower risk of serious sexual side effects, mood changes and other adverse effects. But this risk reduction can vary between different topical formulations.
You can learn more about the differences between topical finasteride vs oral finasteride by visiting our blog post in the XYON Medical Library.
Does topical finasteride have side effects?
All medications come with a potential risk of side effects and topical finasteride is no exception. Compared to oral finasteride, topical finasteride is believed to result in fewer side effects due to less absorption of the drug into the bloodstream. The side effects of topical finasteride are similar to those of oral finasteride and can include: sexual dysfunction, decreased libido and mood changes. This is not an exhaustive list of all the possible adverse effects associated with finasteride use.
We have an article on side effects of finasteride if you'd like to learn more.
What is the success rate of finasteride for hair loss?
One large-scale Japanese study found the response rate to finasteride to be approximately 87% (Sato and Takeda, 2012). However, the success of hair loss treatment can vary between patients. It's important to remember that the success of treatment can be measured by a) amount of hair growth and b) whether hair loss has stabilized. Either result is positive.
Prescription
50% of men will experience hair loss before the age of 50 and 95% of hair loss is treatable.
You can pause or cancel your subscription at any time.






