What is SiloxysSystem™ Gel?
At XYON, we have combined decades of pharmaceutical and clinical expertise to develop exceptional treatments for men experiencing hair loss. SiloxysSystem™, our proprietary liposomal gel, is a sophisticated drug delivery system that can be combined with a medication such as finasteride. Such a treatment is referred to as a compounded medication and is only available with a physician's prescription.
This gel carrier is designed to allow finasteride to be applied to the scalp in a precise and highly targeted way, maximizing exposure of hair follicles within the scalp skin and minimizing exposure of the rest of the body. Our collaboration with leading Italian formulation scientists ensured that SiloxysSystem™ Gel was developed in accordance with the highest quality standards.
Background on finasteride
Oral finasteride has been used to treat androgenetic alopecia (also known as male pattern hair loss) for over 20 years. This medication targets the enzyme 5-alpha reductase, which is involved in the production of the male sex steroid hormone dihydrotestosterone (DHT). The binding of DHT to hair follicle cells is recognized as the cause of hair follicle miniaturization and ultimately, premature hair loss in susceptible individuals (Cranwell & Sinclair, 2016).
Clinical and real-world data have shown that finasteride is effective at reducing levels of DHT, slowing or even halting the progression of hair loss and encouraging hair regrowth (Zito et al., 2022). However, as with most medications, it may come with side effects.
The side effect profile of oral finasteride has been extensively studied. Current data suggests that the majority of side effects are sexual (e.g. erectile dysfunction, decreased libido, decreased ejaculatory volume). However, psychiatric and mood changes have also been reported (Divicarro et al., 2020).
In many cases, these side effects resolve over time. However, in a small subset of individuals presenting with post-finasteride syndrome, side effects can persist even after discontinuing treatment (Pereira & Coelho, 2020).
Why was SiloxysSystem™ Gel developed?
Researchers have attributed the wide-ranging side effects of finasteride to systemic exposure to the medication. When delivered orally or in certain topical formulations, finasteride may reach parts of the body at higher concentrations than might be safely tolerated.
Experience SiloxysSystem™ Gel. It's topical hair loss treatment, elevated.


A patented delivery system designed to deliver the best results.
SiloxysSystem™ Gel was developed to help reduce the risk of systemic exposure. Application of the gel directly to the scalp helps localize the medication. Meanwhile, barrier-forming components within the gel facilitate the controlled release of drug molecules. This controlled release maximizes the distribution of the medication among hair follicle cells.
These properties enable good penetration of finasteride across the top layer of skin (i.e. epidermis) and reduce the passive movement of the medication from the skin into the bloodstream. It is important to remember that not all topical formulas are created equal and that these properties are specific to SiloxysSystem™ Gel.
How was SiloxysSystem™ Gel tested?
Safety and efficacy are our top priority. To ensure that Topical Finasteride, with SiloxysSystem™ Gel performs as expected, clinical studies were conducted by Dr. Victor Hasson, a world-renowned hair restoration specialist. These studies helped assess how a medication such as finasteride is absorbed, distributed, metabolised and excreted (eliminated) when delivered with a carrier such as SiloxysSystem™ Gel.
The two analytical studies performed were a Franz Cell Diffusion Test and Pharmacokinetics (PK) Study.
Franz cell: overview
A Franz Cell Diffusion Test is an in vitro testing method. This means that the test occurs outside the body of a living organism, in a laboratory setting. It is used to determine how much of a medication permeates through a given area of skin over time. This is analogous to absorption into the bloodstream.
The basic Franz Cell consists of three parts: a donor chamber, membrane and receptor chamber. The receptor chamber has a sampling port which allows samples to be retrieved at regular time intervals for analysis.
In our tests, we used a small human skin sample as the membrane to mimic the body’s natural barrier. Three different gel carriers were compared: (1) SiloxysSystem™ Gel, (2) a standard carbopol-based gel and (3) a standard liposomal gel. Each gel was individually combined with the same amount of finasteride (2.5% by weight).
The gels were then individually introduced into the donor chamber of the Franz Cell. Permeation (absorption) of finasteride through the skin membrane was measured over the course of 24 hours. The amount of drug in these samples was further quantified using liquid chromatography and mass spectroscopy, which are two standard analytical techniques.
Franz cell results: measuring skin absorption of finasteride
.jpg?q=75&fit=clip&auto=format&w=3840)
Figure 1 – Permeation amounts of finasteride after 24 hours for three different types of gel carriers
*An approximation of the maximum plasma concentration of finasteride based on known parameters of membrane area, flux (rate of permeation per a given area) and systemic clearance (reference rate of elimination of oral finasteride)
The amount of finasteride that permeated the skin membrane at the end of 24 hours was found to be 0.66 ± 0.24 mcg/cm2 with SiloxysSystem™ Gel, 1.55 ± 0.06 mcg/cm2 with the carbopol-based gel and 4.11 ± 1.50 mcg/cm2 with the standard liposomal gel. A calculation of estimated plasma steady state concentrations was also performed.
What is steady state?
Steady state refers to the point at which the quantity of a medication being eliminated from the body equals the rate at which the same quantity of medication enters the body. This is generally considered the point at which the mediation will have its maximum effect. For some medications, steady state may be achieved within days of starting treatment. For others, this process may take weeks.
Using a standard reference value for systemic clearance (rate of elimination) for oral finasteride, maximum plasma concentrations of the medication were obtained for each of the three gel carriers.
These concentrations were found to be 0.49 ng/mL, 0.74 ng/mL and 2.69 ng/mL for SiloxysSystem™ Gel, the carbopol-based gel and standard liposomal gel, respectively.
Interpretations of Franz cell data
When administered with SiloxysSystem™ Gel technology, permeation of finasteride across the skin was approximately 6-7 times less than that of a typical liposomal carrier over 24 hours. Additionally, in the case of the standard liposomal gel, a bolus effect (delivery of a single large dose of medication over a short time interval) was observed within the first 8 hours of introduction into the donor chamber.
In comparison, when SiloxysSystem™ Gel was used to deliver 2.5% finasteride, a near constant rate of drug delivery was achieved over a 24 hour period. This demonstrates a highly desirable “controlled release” kinetic pattern.
Furthermore, estimations of plasma steady state concentrations of finasteride showed that SiloxysSystem™ Gel technology is associated with the lowest levels of finasteride (0.49 ng/mL) among the three carriers tested. For reference, the plasma steady state concentration of oral finasteride 1 mg (taken once daily) is 9.2 ng/mL, approximately 19 times higher.
This data suggests that when used as a carrier, SiloxysSystem™ Gel can reduce permeation of a medication such as finasteride. The gel’s barrier-forming properties allow for slow and steady release of the medication that is not observed in either the standard carbopol or liposomal gels. This is a highly desirable attribute to have in a delivery system for medication.
PK study: overview
PK studies provide further insight into a medicine’s ADME (absorption, distribution, metabolism and excretion) properties. To assess how much of a medication such as finasteride has entered and remains in the systemic circulation, a series of blood draws is performed on study participants after administration of the medication.
This is done in order to measure the concentration of the medication in the circulation at different time points over a 24-hour period and provides a more accurate representation of the exposure of the body to a medication, compared to a single blood draw at one time point.
Methods
A PK study was performed to assess the effect of administering finasteride using SiloxysSystem™ Gel technology on:
- How much finasteride is absorbed and remains in the bloodstream and
- Blood levels of DHT.
Study eligibility
In order to be eligible for this clinical study, participants needed to be diagnosed with male pattern hair loss. The degree of hair loss was assessed using the Hamilton-Norwood Scale and individuals with more advanced hair loss were included.
Participants were ensured to not be taking finasteride at the time of the study and could not have taken finasteride in the month prior to enrollment. Furthermore, they could not have taken any anabolic steroids, antiandrogen therapies or testosterone replacement in the past 6 months. Scalp skin had to be intact and healthy. Concomitant use of any other topical medications or investigative drug products was not permitted.
Study candidates who met the above criteria were informed of the risks and potential benefits of the treatment. A consent form was dated and signed by each participant prior to voluntarily participating in this study. Each participant was counselled on possible side effects of finasteride and instructed to inform the physician if they experienced any untoward reaction(s) to the medication.
Study procedures
First, a pre-treatment blood draw was performed to obtain a baseline DHT measurement for each study participant. This was repeated two more times at hour 1 and hour 4 after the first blood draw in order to allow each participant to act as his own control.
Immediately following the third DHT measurement at hour 4, study participants applied 2.5% Topical Finasteride with SiloxysSystem™ Gel as instructed by the study investigator, Dr. Victor Hasson. Participants were asked to continue once daily application of the gel every morning for one month. This was to ensure that the drug would reach steady state before the next round of blood draws.
After one month of home treatment with 2.5% Topical Finasteride with SiloxysSystem™ Gel, study participants were brought back to the clinic. A pre-dose blood draw was performed on each participant prior to their morning application (this is known as a trough level).
Following dosing, additional blood draws were performed at hours 1, 2, 4, 8 and 24 to assess post-dose finasteride concentrations, as well as the effect on serum DHT levels. Participants were asked to abstain from strenuous exercise and sexual activity in the 2 days prior to testing to minimize the possibility of transient DHT elevations interfering with measurements.
Interpreting PK test data:
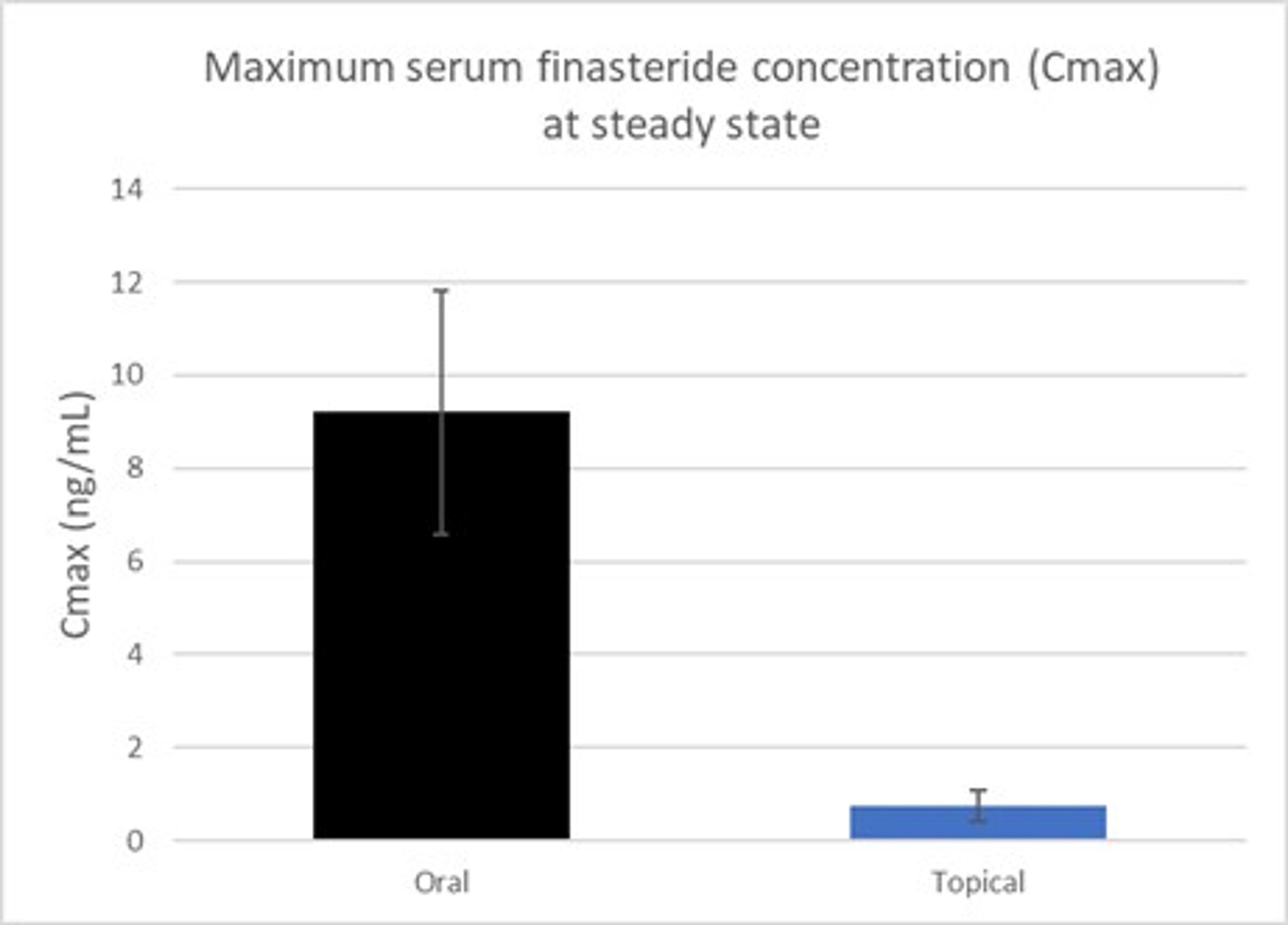
Figure 2 – Mean maximum finasteride serum concentration at steady state
(1) FDA Clinical Pharmacology review of Propecia® NDA 20-788. This was not a head-to-head study
7 participants were enrolled into the study. Application of 2.5% Topical Finasteride with SiloxysSystem™ Gel resulted in a mean plasma concentration of finasteride of 0.74 ± 0.34 ng/mL (Figure 2). This is a markedly lower level of circulating finasteride compared to the reference value of 9.20 ± 2.63 ng/mL seen with the oral pill. Additionally, once steady state was achieved, there was no post-dose spike in finasteride levels over a 24 hour period (Figure 3).
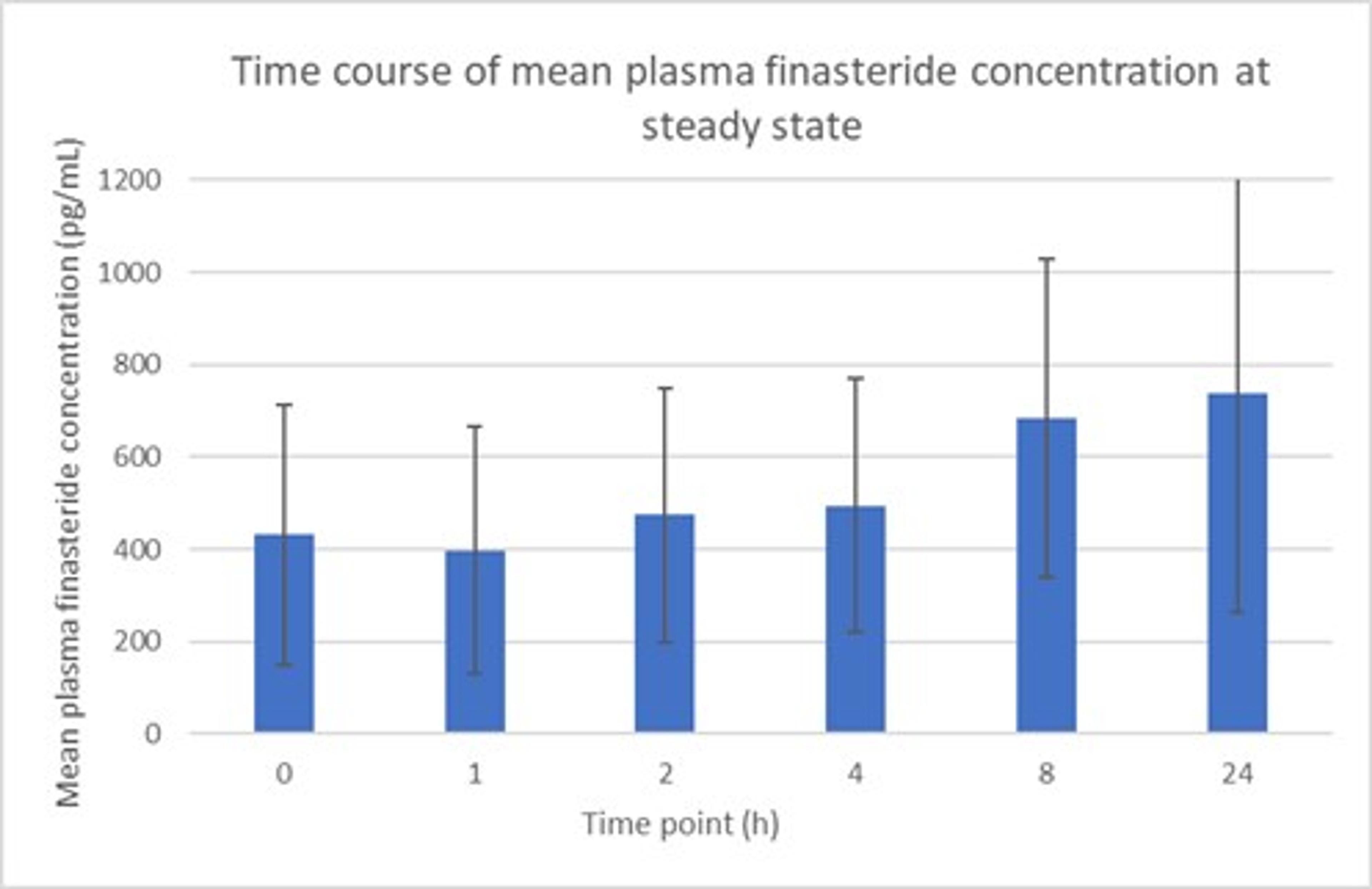
Figure 3 – Mean change in concentration of finasteride at steady state over 24 hours. This was not a head-to-head study
It was also noted that the effect of topical finasteride on serum DHT levels was minimized with SiloxysSystem™ Gel technology. Compared to oral finasteride, suppression of serum DHT levels decreased by 55% (-31 ± 21% with the topical vs. -70 ± 9% with the oral) (Figure 4) and mean plasma DHT concentrations remained fairly constant over the course of 24 hours (Figure 5).
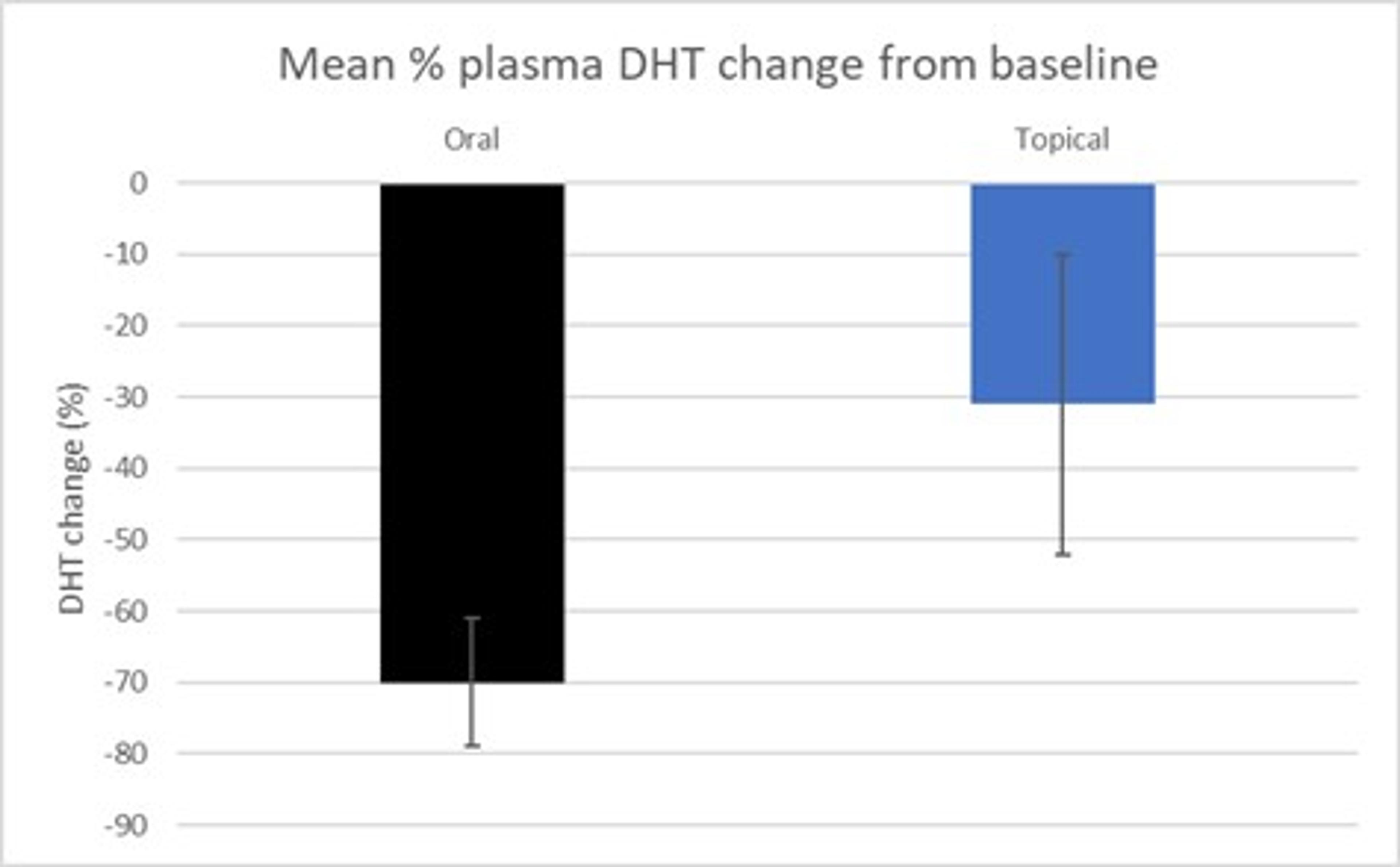
Figure 4 – Mean % change in DHT from baseline
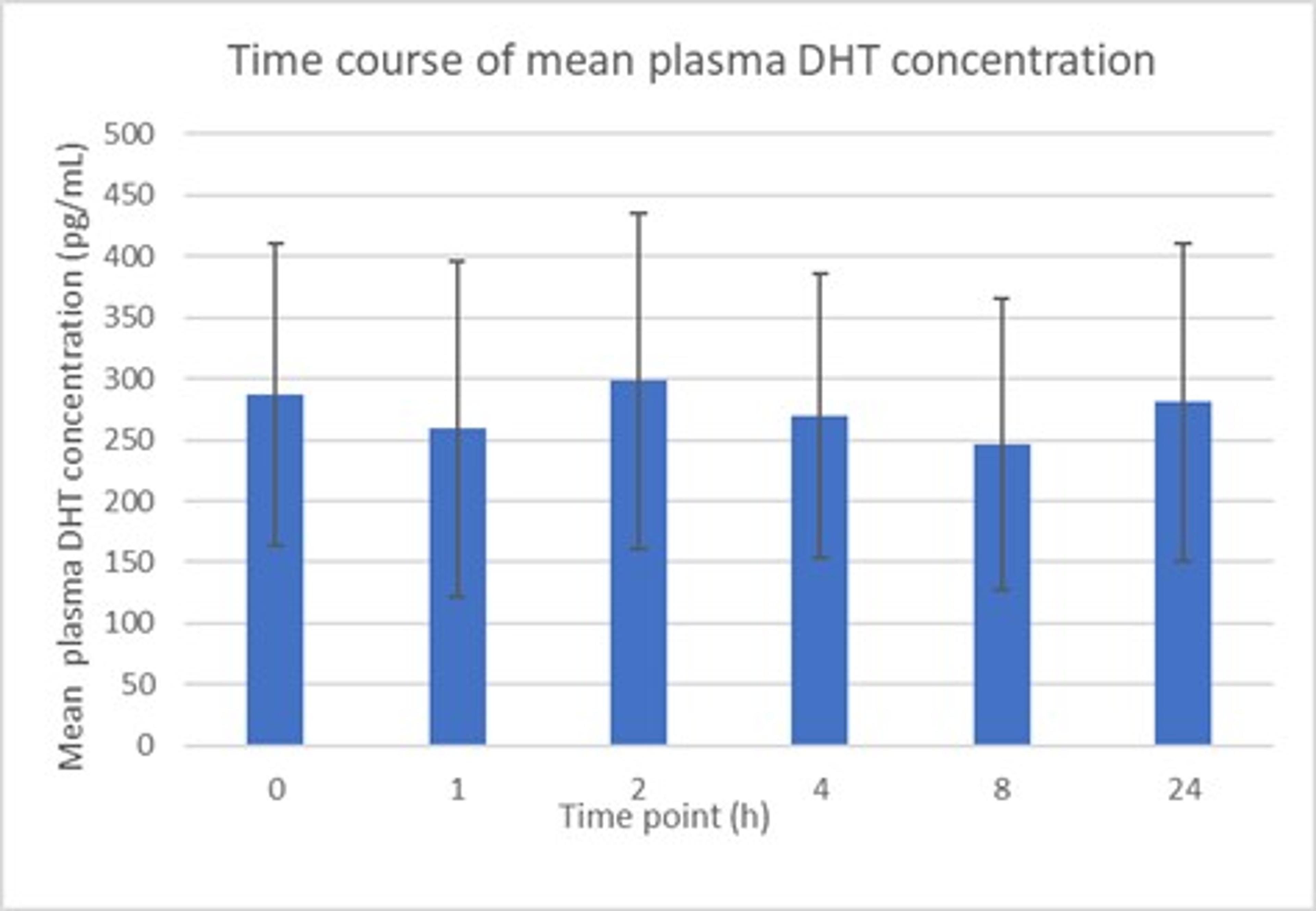
Figure 5 – Mean change in DHT concentration over 24 hours at steady state
XYON clinical trials summary
The trial data suggests that SiloxysSystem™ Gel technology does not completely prevent finasteride from entering systemic circulation. However, it is able to reduce absorption of finasteride into the bloodstream and help minimize the effects of finasteride on blood DHT levels in men. These characteristics may not be present in other topical formulations of finasteride and are distinct from oral finasteride.
In the case of SiloxysSystem™ Gel, the absence of a sudden post-dose increase in blood levels of finasteride reinforces the notion that the gel forms a barrier across skin to enable slow, controlled release of the medication over time. This controlled release most likely maximizes the interaction between hair follicle cells and finasteride.
Furthermore, the stability of post-dose blood DHT levels suggests that reduced finasteride absorption avoids broad disruption of androgen levels. This could play a role in decreasing the incidence of sexual side effects associated with finasteride use.
Although the small sample size of 7 participants was a limitation in this study, none of the participants reported side effects of any kind while using this topical treatment. There was no evidence of any local or systemic adverse effects and the treatment was well tolerated.
Topical finasteride with SiloxysSystem™ Gel: Takeaway
Our topical finasteride study results show that SiloxysSystem™ Gel is a truly unique liposomal carrier. The barrier-forming gel was specifically designed to achieve steady delivery of a medication such as finasteride without compromising its efficacy.
The technology limits the absorption of the compounded medication finasteride, minimizing its effect on blood DHT levels and potentially leading to a reduced risk of sexual dysfunction. This kind of precision targeted treatment may be a critical step to enhancing the safety of 5-alpha reductase inhibitors and other pharmaceuticals.
Acknowledgements
We would like to thank the participants of this study and the clinic staff at the office of Dr. Hasson for their generous time commitment. We would also like to thank Dr. Hasson for leading these investigations.
We also wish to thank Dr. Paola Minghetti (Università degli Studi di Milano) and colleagues for their work on the Franz Cell skin permeation studies presented in this report.




