

Hair loss isn’t one-size-fits-all. Explore our collection of men's hair solutions designed to support hair and scalp health.
Three steps to better hair
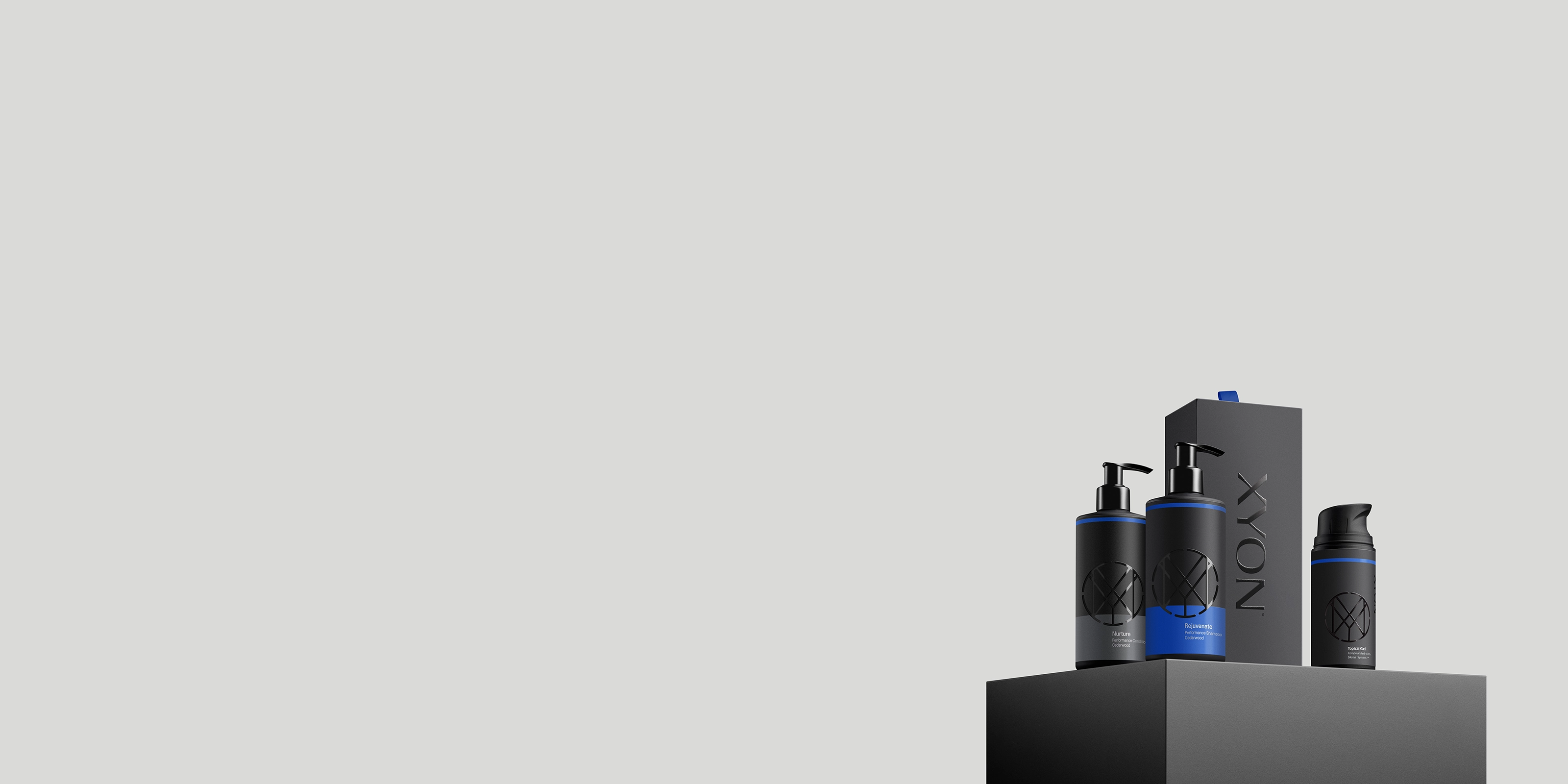
Each of our men's hair care products are formulated to support hair regrowth by targeting DHT and creating an optimal scalp environment for stronger, thicker hair. Use them together for best results.

Cleanse
XYON's DHT-Blocking Shampoo features a blend of natural hair-thickening ingredients to combat causes of male pattern baldness. Cleanses without stripping moisture for softer, healthier hair.

Condition
Follow the shampoo with XYON's DHT-Blocking Conditioner, formulated with thickening and strengthening ingredients. Moisturizes and softens without weighing hair down.

Defend
Finish with XYON's Defend Hair & Scalp Serum. Designed to hydrate the scalp and stimulate hair growth in one easy-to-use, lightweight, fast-absorbing formula.
Sourced from nature, selected for performance
Meet our DHT-blocking ingredients

Saw Palmetto
Naturally blocks DHT to prevent hair follicle shrinkage and promote fuller, thicker hair growth.

Ginseng Root
Promotes follicle health by increasing circulation to the scalp and countering environmental damage.

Rosemary Oil
Enhances circulation to boost hair growth. Antioxidants and anti-inflammatories fight aging and optimize scalp health.

Biotin
Supports hair growth by boosting keratin production, a protein needed for hair structure and strength.

Capixyl™ peptide complex
Helps strengthen the attachment between hairs and the scalp while increasing hair thickness.

Caffeine
Promotes circulation to the scalp, increasing nutrient and oxygen delivery to improve hair growth.
Get more from your hair care
Make XYON part of your routine
Whether you’re looking to reduce breakage, fight loss, or encourage healthier hair regrowth, our hair care products are designed to complement any of our prescription treatments. Use them alone or together with your prescription to get the results that you want.
Explore prescription solutions
Receive expert care and treatment recommendations by starting a virtual consultation with one of our licensed physicians.
with SiloxysSystem™ Gel
Safety Information
Before you start using finasteride and each time you get a refill, please review this important safety information. This information may be updated. It does not replace discussing your medical condition or treatment with your doctor or healthcare provider.
What is the FDA approved use of finasteride?
- Finasteride is FDA-approved as an oral medication to treat benign prostatic hyperplasia (Proscar) and androgenetic alopecia (Propecia) in men.
- Finasteride has not been FDA-approved for topical use in men or women. However, it may be prescribed off-label in topical compounded formulations to treat hair loss. Please note that oral finasteride has also not been approved for use in women, for any indication.
What is topical finasteride?
Topical finasteride is a compounded prescription medication that may be used for the treatment of male and female pattern hair loss (androgenetic alopecia). This compounded treatment is not approved by the FDA. The FDA does not verify the safety or effectiveness of compounded medications. Topical finasteride may only be prescribed after an online consultation with a licensed physician through the XYON Health platform.
Data from small scale and case studies suggests that topical finasteride may be an effective treatment for hair loss, with a potentially lower risk of side effects compared to the oral medication as a result of reduced systemic absorption.
What should I tell my healthcare provider before taking finasteride?
Please inform your doctor or healthcare provider if any of the following apply:
- You have a personal or family history of prostate cancer, breast cancer
- You have prostate or urinary symptoms such as reduced urine flow, difficulty urinating or pain
- You have a history of any serious mental health conditions (e.g., depression, anxiety, suicidal thoughts)
- You are pregnant, trying to get pregnant, or are breastfeeding
Who should not take finasteride?
You should not take finasteride if you:
- Have ever had an allergic reaction to finasteride or any other ingredients in the treatment
- Finasteride should not be used by women who are pregnant, may be pregnant or are breastfeeding
- Finasteride should not be used by infants or children
What are the possible side effects of finasteride?
The following side effects have been reported with finasteride use:
- Breast tenderness and enlargement. You should inform your doctor or healthcare provider if you notice any breast lumps, pain or nipple discharge
- Depression, anxiety, brain fog, suicidal ideation
- Decreased libido (sex drive)
- Allergic reactions (e.g., rash, itching, hives and swelling of the lips, tongue, throat, and face)
- Problems with ejaculation
- Testicular pain
- Erectile dysfunction (difficulty achieving or maintaining an erection)
- Male infertility and/or poor semen quality
Possible long-term side effects have also been reported with finasteride use.
Please note that finasteride has only been formally studied in male patients, so the side effect profile above is specific to clinical studies in men. Preliminary data on the use of finasteride in female patients has shown the following possible side effects:
- Decreased libido (sex drive)
- Breast tenderness and enlargement
- Mood changes (e.g., depression)
- Fatigue
- Muscle soreness
- Weight gain
There is some evidence that finasteride can increase the risk of some breast and prostate cancers.
When used off label in a topical form, systemic side effects of finasteride are expected to be less frequent/severe. The following is a list of possible side effects associated with the use of topical finasteride:
- Itching
- Irritation
- Dry and flaky scalp
- Increased scalp oiliness
The above lists do not represent all potential side effects, medication interactions or study data relating to the safety of finasteride. It is important to talk to your doctor, healthcare provider and/or pharmacist if you are experiencing any side effects. A comprehensive review of the potential safety risks of using finasteride can be found at this link: https://pubmed.ncbi.nlm.nih.gov/27672412/.
If you are experiencing a medical emergency or allergic reaction, call 911 or seek immediate medical attention. You are encouraged to report negative side effects of prescription products to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. Additional safety information can be found here, in the Digital Prescriber’s Reference for finasteride: https://www.pdr.net/drug-summary/?drugLabelId=378.
with SiloxysSystem™ Gel
Safety Information
Before you start using finasteride and minoxidil and each time you get a refill, please review this important safety information. This information may be updated. It does not replace discussing your medical condition or treatment with your doctor or healthcare provider. Safety information for both finasteride and minoxidil is contained within this document. Please take care to review this entire document.
What is the FDA approved use of finasteride?
- Finasteride is FDA-approved as an oral medication to treat benign prostatic hyperplasia (Proscar) and androgenetic alopecia (Propecia) in men.
- Finasteride has not been FDA-approved for topical use in men or women. However, it may be prescribed off-label in topical compounded formulations to treat hair loss. Please note that oral finasteride has also not been approved for use in women, for any indication.
What is the FDA approved use of minoxidil?
- Topical minoxidil is FDA approved at concentrations of 2% and 5% as topical formulations to treat hair loss in women and men (Rogaine®). All other topical compounded strengths of minoxidil are considered off-label uses.
- Oral minoxidil is FDA-approved for the treatment of symptomatic high blood pressure. Low-dose oral minoxidil may be prescribed off-label to treat hair loss.
What is topical finasteride and minoxidil?
Topical finasteride and minoxidil is a compounded prescription medication used for the treatment of male and female pattern hair loss (androgenetic alopecia). This compounded treatment is not approved by the FDA. The FDA does not verify the safety or effectiveness of compounded medications. Topical finasteride and minoxidil may only be prescribed after an online consultation with a licensed physician through the XYON Health platform.
Data from small scale and case studies suggests that taking finasteride in combination with minoxidil may increase the effectiveness of hair loss treatment in some patients. This may be due to different mechanisms of action that help address the various causes of pattern hair loss.
What should I tell my healthcare provider before taking finasteride and minoxidil?
Please inform your doctor or healthcare provider if any of the following apply:
- You have a personal or family history of prostate cancer, breast cancer
- You have prostate or urinary symptoms such as reduced urine flow, difficulty urinating or pain
- You have a history of heart conditions (e.g., high blood pressure, heart failure, previous heart attack, pericarditis, abnormal heart rhythm, chest pain/angina)
- You have a history of vascular conditions (e.g., stroke, severe lower leg swelling, pulmonary hypertension)
- You have a history of liver or kidney disease or dysfunction
- You have a history of any serious mental health conditions (e.g., depression, anxiety, suicidal thoughts)
- You are pregnant, trying to get pregnant, or are breastfeeding
You should be aware that minoxidil has the potential to be toxic to some animals. Please ensure that pets do not come into contact with minoxidil or lick your hands after application.
Who should not take finasteride and minoxidil?
You should not take finasteride and minoxidil if you:
- Have ever had an allergic reaction to finasteride, minoxidil or any other ingredients in the treatment
- Have a history of liver disease or dysfunction
- Finasteride and minoxidil should not be used by women who are pregnant, may be pregnant or are breastfeeding
- Finasteride and minoxidil should not be used by infants or children
Black box warning: Serious cardiac effects associated with oral minoxidil
- Oral minoxidil has a black box warning because the medication is a powerful antihypertensive agent. Serious cardiovascular side effects have been observed in clinical trials where the drug has been used to treat symptomatic high blood pressure. When used off-label for hair loss, oral minoxidil is typically dosed at less than 5mg to help reduce the likelihood of experiencing an adverse cardiovascular event
- Oral minoxidil can cause pericardial effusion, which can progress to a condition called tamponade which increases pressure on the heart and keeps it from beating properly. This can result in sharp chest pain and difficulty breathing. Seek emergency treatment if this occurs
- Chest pain warning: Minoxidil can increase your heart rate and cause or worsen chest pain. If you have new or worsening pain in the chest, arm, or shoulders, tell your doctor right away or go to the nearest emergency department.
- Heart function warning: Minoxidil can cause poor heart function or worsen existing heart problems like heart failure. When tested on animals, minoxidil caused several kinds of lesions of the heart muscle as well as other adverse heart effects. Patients with malignant hypertension and those already receiving guanethidine should be hospitalized when minoxidil is first administered so that they can be monitored to avoid too rapid or large decreases in blood pressure
What are the possible side effects of finasteride and minoxidil?
The following side effects have been reported with finasteride use:
- Breast tenderness and enlargement. You should inform your doctor or healthcare provider if you notice any breast lumps, pain or nipple discharge
- Depression, anxiety, brain fog, suicidal ideation
- Decrease libido (sex drive)
- Allergic reactions (e.g., rash, itching, hives and swelling of the lips, tongue, throat, and face)
- Problems with ejaculation
- Testicular pain
- Erectile dysfunction (difficulty achieving or maintaining an erection)
- Male infertility and/or poor semen quality
Possible long-term side effects have also been reported with finasteride use.
Please note that finasteride has only been formally studied in male patients, so the side effect profile above is specific to clinical studies in men. Preliminary data on the use of finasteride in female patients has shown the following possible side effects:
- Decreased libido (sex drive)
- Breast tenderness and enlargement
- Mood changes (e.g., depression)
- Fatigue
- Muscle soreness
- Weight gain
There is some evidence that finasteride can increase the risk of some breast and prostate cancers.
When used off label in a topical form, systemic side effects of finasteride and minoxidil are expected to be less frequent/severe. The following is a list of possible side effects associated with the use of topical finasteride:
- Itching
- Irritation
- Dry and flaky scalp
- Increased scalp oiliness
The following side effects have been reported with topical minoxidil use:
- Itchy scalp or skin rash
- Dryness, scaling, or flakiness
- Redness or irritation at the application site
The above lists do not represent all potential side effects, medication interactions or study data relating to the safety of finasteride and minoxidil. It is important to talk to your doctor, healthcare provider and/or pharmacist if you are experiencing any side effects. A comprehensive review of the potential safety risks of using finasteride and other similar medications can be found at this link: https://pubmed.ncbi.nlm.nih.gov/27672412/. A review of minoxidil, its clinical applications and safety can be found at this link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6691938/. If you are experiencing a medical emergency or allergic reaction, call 911 or seek immediate medical attention. You are encouraged to report negative side effects of prescription products to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. Additional safety information can be found here, in the Digital Prescriber’s Reference for finasteride: https://www.pdr.net/drug-summary/?drugLabelId=378 and Digital Prescriber’s Reference for oral minoxidil: https://www.pdr.net/drug-summary/?drugLabelId=774.
with SiloxysSystem™ Gel
Safety Information
Before you start using dutasteride and each time you get a refill, please review this important safety information. This information may be updated. It does not replace discussing your medical condition or treatment with your doctor or healthcare provider.
What is the FDA approved use of dutasteride?
Dutasteride is FDA approved as an oral medication to treat benign prostatic hyperplasia (Proscar) in men. Dutasteride has not been FDA approved for topical use or specifically for use in women, but it may be prescribed off-label in topical compounded formulations for hair loss.
You can read more about some of the study data on dutasteride use in women by visiting this link:
What is topical dutasteride?
Topical dutasteride is a compounded prescription medication used for the treatment of male and female pattern hair loss (androgenetic alopecia). This compounded treatment is not approved by the FDA and is only available if prescribed after an online consultation with a licensed physician through the XYON Health platform. Data from small scale and case studies have shown that topical dutasteride may help reduce serum DHT levels with a lower incidence of side effects (e.g., sexual dysfunction) that have been reported with the oral versions of this class of medications.
What should I tell my healthcare provider before taking dutasteride?
Please inform your doctor or healthcare provider if any of the following apply:
- You have a personal or family history of prostate cancer, breast cancer
- You have prostate or urinary symptoms such as reduced urine flow, difficulty urinating or pain
- You have a history of liver or kidney disease or dysfunction
- You have a history of any serious mental health conditions
- You are pregnant, trying to get pregnant, or are breastfeeding
Who should not take dutasteride?
You should not take dutasteride if you:
- Have ever had an allergic reaction to dutasteride or any other ingredients in the treatment
- Have a history of liver disease or dysfunction
- Dutasteride should not be used by women who are pregnant, may be pregnant or are breastfeeding
- Dutasteride should not be used by infants or children
What are the possible side effects of dutasteride?
The following side effects have been reported with dutasteride use:
- Breast tenderness and enlargement. You should inform your doctor or healthcare provider if you notice any breast lumps, pain or nipple discharge
- Depression, anxiety, brain fog, suicidal ideation
- Decrease libido (sex drive)
- Allergic reactions (e.g., rash, itching, hives and swelling of the lips, tongue, throat, and face)
- Problems with ejaculation
- Testicular pain
- Erectile dysfunction (difficulty achieving or maintaining an erection)
- Male infertility and/or poor semen quality
Please note that dutasteride has only been formally studied in male patients so the side effect profile above is specific to clinical studies in men.
Preliminary data on the use of dutasteride in female patients has shown the following possible side effects:
- Decreased libido (sex drive)
- Breast tenderness and enlargement
- Mood changes (e.g., depression)
- Fatigue
- Muscle soreness
- Weight gain
There is some evidence that dutasteride can increase the risk of some breast and prostate cancers. When used off label in a topical form, systemic side effects of dutasteride are expected to be less frequent/severe, although no formal clinical studies have been done to confirm the safety and efficacy of topical dutasteride. The following is a list of possible side effects associated with the use of topical dutasteride:
- Itching
- Irritation
- Dry and flaky scalp
- Increased scalp oiliness
The above does not represent all potential side effects, medication interactions or study data relating to the safety of dutasteride. It is important to talk to your doctor, healthcare provider and/or pharmacist if you are experiencing any side effects. If you are experiencing a medical emergency or allergic reaction, call 911 or seek immediate medical attention. You are encouraged to report negative side effects of prescription products to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
You can refer to the FDA prescribing information as well as the Prescriber’s Digital Reference (PDR) for comprehensive overviews of dutasteride.
Safety Information
Before you start using finasteride and minoxidil and each time you get a refill, please review this important safety information. This information may be updated. It does not replace discussing your medical condition or treatment with your doctor or healthcare provider. Safety information for both finasteride and minoxidil is contained within this document. Please take care to review this entire document.
What is the FDA approved use of finasteride?
- Finasteride is FDA-approved as an oral medication to treat benign prostatic hyperplasia (Proscar) and androgenetic alopecia (Propecia) in men.
- Finasteride has not been FDA-approved for topical use in men or women. However, it may be prescribed off-label in topical compounded formulations to treat hair loss. Please note that oral finasteride has also not been approved for use in women, for any indication.
What is the FDA approved use of minoxidil?
- Topical minoxidil is FDA approved at concentrations of 2% and 5% as topical formulations to treat hair loss in women and men (Rogaine®). All other topical compounded strengths of minoxidil are considered off-label uses.
- Oral minoxidil is FDA-approved for the treatment of symptomatic high blood pressure. Low-dose oral minoxidil may be prescribed off-label to treat hair loss.
What is topical finasteride and minoxidil?
Topical finasteride and minoxidil is a compounded prescription medication used for the treatment of male and female pattern hair loss (androgenetic alopecia). This compounded treatment is not approved by the FDA. The FDA does not verify the safety or effectiveness of compounded medications. It may only be prescribed after an online consultation with a licensed physician through the XYON Health platform.
Data from small scale and case studies suggests that taking finasteride in combination with minoxidil may increase the effectiveness of hair loss treatment in some patients. This may be due to different mechanisms of action that help address the various causes of pattern hair loss.
What should I tell my healthcare provider before taking finasteride and minoxidil?
Please inform your doctor or healthcare provider if any of the following apply:
- You have a personal or family history of prostate cancer, breast cancer
- You have prostate or urinary symptoms such as reduced urine flow, difficulty urinating or pain
- You have a history of heart conditions (e.g., high blood pressure, heart failure, previous heart attack, pericarditis, abnormal heart rhythm, chest pain/angina)
- You have a history of vascular conditions (e.g., stroke, severe lower leg swelling, pulmonary hypertension)
- You have a history of liver or kidney disease or dysfunction
- You have a history of any serious mental health conditions (e.g., depression, anxiety, suicidal thoughts)
- You are pregnant, trying to get pregnant, or are breastfeeding
You should be aware that minoxidil has the potential to be toxic to some animals. Please ensure that pets do not come into contact with minoxidil or lick your hands after application.
Who should not take finasteride and minoxidil?
You should not take finasteride and minoxidil if you:
- Have ever had an allergic reaction to finasteride, minoxidil or any other ingredients in the treatment
- Have a history of liver disease or dysfunction
- Finasteride and minoxidil should not be used by women who are pregnant, may be pregnant or are breastfeeding
- Finasteride and minoxidil should not be used by infants or children
Black box warning: Serious cardiac effects associated with oral minoxidil
- Oral minoxidil has a black box warning because the medication is a powerful antihypertensive agent. Serious cardiovascular side effects have been observed in clinical trials where the drug has been used to treat symptomatic high blood pressure. When used off-label for hair loss, oral minoxidil is typically dosed at less than 5mg to help reduce the likelihood of experiencing an adverse cardiovascular event
- Oral minoxidil can cause pericardial effusion, which can progress to a condition called tamponade which increases pressure on the heart and keeps it from beating properly. This can result in sharp chest pain and difficulty breathing. Seek emergency treatment if this occurs
- Chest pain warning: Minoxidil can increase your heart rate and cause or worsen chest pain. If you have new or worsening pain in the chest, arm, or shoulders, tell your doctor right away or go to the nearest emergency department.
- Heart function warning: Minoxidil can cause poor heart function or worsen existing heart problems like heart failure. When tested on animals, minoxidil caused several kinds of lesions of the heart muscle as well as other adverse heart effects. Patients with malignant hypertension and those already receiving guanethidine should be hospitalized when minoxidil is first administered so that they can be monitored to avoid too rapid or large decreases in blood pressure
What are the possible side effects of finasteride and minoxidil?
The following side effects have been reported with finasteride use:
- Breast tenderness and enlargement. You should inform your doctor or healthcare provider if you notice any breast lumps, pain or nipple discharge
- Depression, anxiety, brain fog, suicidal ideation
- Decrease libido (sex drive)
- Allergic reactions (e.g., rash, itching, hives and swelling of the lips, tongue, throat, and face)
- Problems with ejaculation
- Testicular pain
- Erectile dysfunction (difficulty achieving or maintaining an erection)
- Male infertility and/or poor semen quality
Possible long-term side effects have also been reported with finasteride use.
Please note that finasteride has only been formally studied in male patients, so the side effect profile above is specific to clinical studies in men. Preliminary data on the use of finasteride in female patients has shown the following possible side effects:
- Decreased libido (sex drive)
- Breast tenderness and enlargement
- Mood changes (e.g., depression)
- Fatigue
- Muscle soreness
- Weight gain
There is some evidence that finasteride can increase the risk of some breast and prostate cancers.
When used off label in a topical form, systemic side effects of finasteride and minoxidil are expected to be less frequent/severe. The following is a list of possible side effects associated with the use of topical finasteride:
- Itching
- Irritation
- Dry and flaky scalp
- Increased scalp oiliness
The following side effects have been reported with topical minoxidil use:
- Itchy scalp or skin rash
- Dryness, scaling, or flakiness
- Redness or irritation at the application site
The above lists do not represent all potential side effects, medication interactions or study data relating to the safety of finasteride and minoxidil. It is important to talk to your doctor, healthcare provider and/or pharmacist if you are experiencing any side effects. A comprehensive review of the potential safety risks of using finasteride and other similar medications can be found at this link: https://pubmed.ncbi.nlm.nih.gov/27672412/. A review of minoxidil, its clinical applications and safety can be found at this link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6691938/. If you are experiencing a medical emergency or allergic reaction, call 911 or seek immediate medical attention. You are encouraged to report negative side effects of prescription products to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. Additional safety information can be found here, in the Digital Prescriber’s Reference for finasteride: https://www.pdr.net/drug-summary/?drugLabelId=378 and Digital Prescriber’s Reference for oral minoxidil: https://www.pdr.net/drug-summary/?drugLabelId=774.
Safety Information
Before you start using minoxidil and each time you get a refill, please review this important safety information. This information may be updated. It does not replace discussing your medical condition or treatment with your doctor or healthcare provider.
What is the FDA-approved use of minoxidil?
- Topical minoxidil is FDA approved at concentrations of 2% and 5% as topical formulations to treat hair loss in women and men (Rogaine®). All other topical compounded strengths of minoxidil are considered off-label uses.
- Oral minoxidil is FDA-approved for the treatment of symptomatic high blood pressure.
- Oral minoxidil is not FDA-approved to treat hair loss, but may be prescribed off-label for this condition.
- Oral minoxidil compounded with Vitamin D is not FDA-approved to treat hair loss.
What should I tell my healthcare provider before taking minoxidil?
Please inform your doctor or healthcare provider if any of the following apply:
- You have a history of heart conditions (e.g., high blood pressure, heart failure, previous heart attack, pericarditis, abnormal heart rhythm, chest pain/angina)
- You have a history of vascular conditions (e.g., stroke, severe lower leg swelling, pulmonary hypertension)
- You have a history of liver or kidney disease or dysfunction
- You have a history of any serious mental health conditions
- You are pregnant, trying to get pregnant, or are breastfeeding
- You are currently taking a Vitamin D supplement*
- You have high blood levels of calcium*
*applies to compounded oral minoxidil with Vitamin D
You should be aware that minoxidil has the potential to be toxic to some animals. Please ensure that pets do not come into contact with minoxidil or lick your hands after application.
It is rare for Vitamin D to cause side effects at the prescribed doses. If you are taking a Vitamin D supplement, consult your doctor before taking compounded oral minoxidil with Vitamin D.
Who should not take minoxidil?
You should not take minoxidil if you:
- Have ever had an allergic reaction to minoxidil
- Have a history of liver disease or dysfunction
- Have high blood levels of calcium*
- Minoxidil should not be used by women who are pregnant, may be pregnant or are breastfeeding
- Minoxidil should not be used by infants or children
Black box warning: Serious cardiac effects associated with oral minoxidil
- Oral minoxidil has a black box warning because the medication is a powerful antihypertensive agent. Serious cardiovascular side effects have been observed in clinical trials where the drug has been used to treat symptomatic high blood pressure. When used off-label for hair loss, oral minoxidil is typically dosed at less than 5mg to help reduce the likelihood of experiencing an adverse cardiovascular event.
- Oral minoxidil can cause pericardial effusion, which can progress to a condition called tamponade which increases pressure on the heart and keeps it from beating properly. This can result in sharp chest pain and difficulty breathing. Seek emergency treatment if this occurs.
- Chest pain warning: Minoxidil can increase your heart rate and cause or worsen chest pain. If you have new or worsening pain in the chest, arm, or shoulders, tell your doctor right away or go to the nearest emergency department.
- Heart function warning: Minoxidil can cause poor heart function or worsen existing heart problems like heart failure. When tested on animals, minoxidil caused several kinds of lesions of the heart muscle as well as other adverse heart effects. Patients with malignant hypertension and those already receiving guanethidine should be hospitalized when minoxidil is first administered so that they can be monitored to avoid too rapid or large decreases in blood pressure.
What are the possible side effects of minoxidil?
The following side effects have been reported with oral and topical minoxidil use:
- A temporary increase in shedding/hair loss in the first weeks of use
- Itchy scalp or skin rash
- Dryness, scaling, or flakiness of the scalp
- Redness or irritation at the application site
- Unwanted facial or body hair growth (especially in women)
- Dizziness or light-headedness
- Rapid or irregular heartbeat
- Chest pain or palpitations
- Swelling of hands, feet, or face
The above list does not represent all potential side effects, medication interactions or study data relating to the safety of minoxidil. It is important to talk to your doctor, healthcare provider and/or pharmacist if you are experiencing any side effects.
A comprehensive review of minoxidil, its clinical applications and safety can be found at this link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6691938/.
If you are experiencing a medical emergency or allergic reaction, call 911 or seek immediate medical attention.
You are encouraged to report negative side effects of prescription products to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Additional safety information can be found here, in the Digital Prescriber’s Reference for oral minoxidil: https://www.pdr.net/drug-summary/?drugLabelId=774.
Safety Information
Before you start using finasteride and each time you get a refill, please review this important safety information. This information may be updated. It does not replace discussing your medical condition or treatment with your doctor or healthcare provider.
What is the FDA approved use of finasteride?
- Finasteride is FDA-approved as an oral medication to treat benign prostatic hyperplasia (Proscar) and androgenetic alopecia (Propecia) in men.
- Finasteride has not been FDA-approved for topical use in men or women. However, it may be prescribed off-label in topical compounded formulations to treat hair loss. Please note that oral finasteride has also not been approved for use in women, for any indication.
What should I tell my healthcare provider before taking finasteride?
Please inform your doctor or healthcare provider if any of the following apply:
- You have a personal or family history of prostate cancer, breast cancer
- You have prostate or urinary symptoms such as reduced urine flow, difficulty urinating or pain
- You have a history of any serious mental health conditions
- You are pregnant, trying to get pregnant, or are breastfeeding
Who should not take finasteride?
You should not take finasteride if you:
- Have ever had an allergic reaction to finasteride or any other ingredients in the treatment
- Finasteride should not be used by women who are pregnant, may be pregnant or are breastfeeding
- Finasteride should not be used by infants or children
What are the possible side effects of finasteride?
The following side effects have been reported with finasteride use:
- Breast tenderness and enlargement. You should inform your doctor or healthcare provider if you notice any breast lumps, pain or nipple discharge
- Depression, anxiety, brain fog, suicidal ideation
- Decreased libido (sex drive)
- Allergic reactions (e.g., rash, itching, hives and swelling of the lips, tongue, throat, and face)
- Problems with ejaculation
- Testicular pain
- Erectile dysfunction (difficulty achieving or maintaining an erection)
- Male infertility and/or poor semen quality
Please note that finasteride has only been formally studied in male patients, so the side effect profile above is specific to clinical studies in men. Preliminary data on the use of finasteride in female patients has shown the following possible side effects:
- Decreased libido (sex drive)
- Breast tenderness and enlargement
- Mood changes (e.g., depression)
- Fatigue
- Muscle soreness
- Weight gain
There is some evidence that finasteride can increase the risk of some breast and prostate cancers.
The above lists do not represent all potential side effects, medication interactions or study data relating to the safety of finasteride. It is important to talk to your doctor, healthcare provider and/or pharmacist if you are experiencing any side effects. A comprehensive review of the potential safety risks of using finasteride can be found at this link: https://pubmed.ncbi.nlm.nih.gov/27672412/.
If you are experiencing a medical emergency or allergic reaction, call 911 or seek immediate medical attention. You are encouraged to report negative side effects of prescription products to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. Additional safety information can be found here, in the Digital Prescriber’s Reference for finasteride: https://www.pdr.net/drug-summary/?drugLabelId=378.








